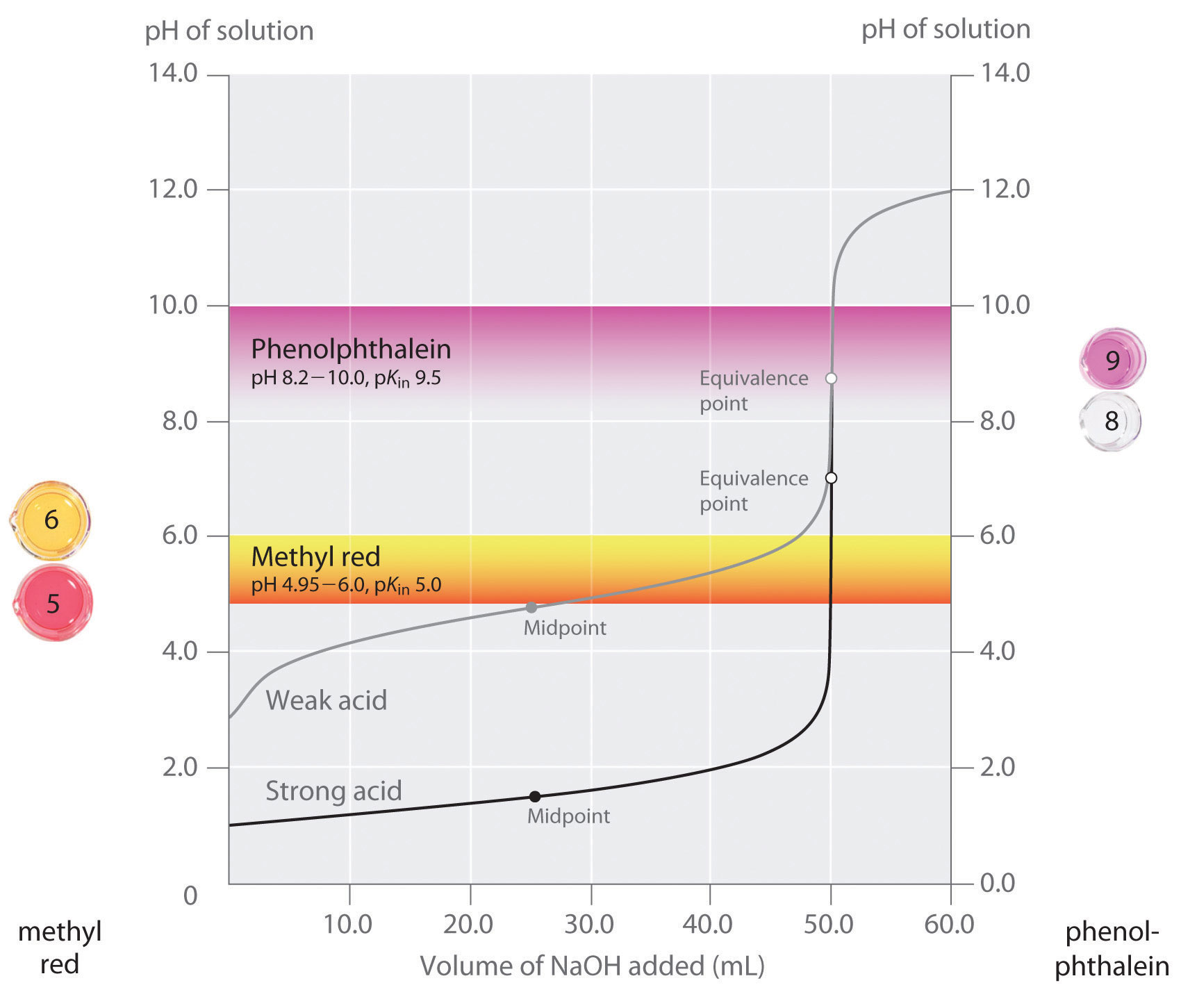
Titration Of Indicators . Indicators are substances whose solutions change color due to changes in ph. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. The graph shows the results obtained using two indicators (methyl red and. They are usually weak acids or bases, but their conjugate base or acid. The ph range of phenolphthalein is about 8.3 to 10.0, but the. This page assumes that you know. Some plants contain coloring agents that. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In the strong acid titration,.
from saylordotorg.github.io
Some plants contain coloring agents that. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know. Indicators are substances whose solutions change color due to changes in ph. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. They are usually weak acids or bases, but their conjugate base or acid. The graph shows the results obtained using two indicators (methyl red and. In the strong acid titration,. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. The ph range of phenolphthalein is about 8.3 to 10.0, but the.
AcidBase Titrations
Titration Of Indicators The ph range of phenolphthalein is about 8.3 to 10.0, but the. In the strong acid titration,. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. Some plants contain coloring agents that. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. The ph range of phenolphthalein is about 8.3 to 10.0, but the. They are usually weak acids or bases, but their conjugate base or acid. The graph shows the results obtained using two indicators (methyl red and.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Of Indicators The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. They are usually weak acids or bases, but their conjugate base. Titration Of Indicators.
From saylordotorg.github.io
AcidBase Titrations Titration Of Indicators The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. They are usually weak acids or bases, but their conjugate base. Titration Of Indicators.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Titration Of Indicators The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Indicators are substances whose solutions change color due to changes in ph. Indicators are therefore only. Titration Of Indicators.
From saylordotorg.github.io
AcidBase Titrations Titration Of Indicators Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. This page assumes that you know. In the strong acid titration,. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl. Titration Of Indicators.
From www.shutterstock.com
AcidBase Titration Setup ,Phenolphthalein Indicator Vector 253299010 Titration Of Indicators In the strong acid titration,. The ph range of phenolphthalein is about 8.3 to 10.0, but the. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and. The two most common indicators that are used in titrations are methyl orange and. Titration Of Indicators.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Titration Of Indicators Indicators are substances whose solutions change color due to changes in ph. In the strong acid titration,. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. Some plants contain coloring agents that. The titration curves shown in figure 14.20 illustrate the choice of a. Titration Of Indicators.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Of Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The ph range of phenolphthalein is about 8.3 to 10.0, but the. They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances. Titration Of Indicators.
From ar.inspiredpencil.com
Titration Diagram Titration Of Indicators In the strong acid titration,. The graph shows the results obtained using two indicators (methyl red and. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. Some plants contain coloring agents that. The two most common indicators that are used in titrations are methyl orange and. Titration Of Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Of Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Some plants contain coloring agents that. The ph range of phenolphthalein is about 8.3 to 10.0, but the. They are usually weak acids or bases, but their conjugate base or acid. In the strong acid titration,. Indicators are therefore only useful in the. Titration Of Indicators.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Of Indicators They are usually weak acids or bases, but their conjugate base or acid. In the strong acid titration,. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. The graph. Titration Of Indicators.
From www.science-revision.co.uk
Titrations Titration Of Indicators The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The ph range of phenolphthalein is about 8.3 to 10.0, but the. The graph shows the results obtained using two indicators (methyl red and. In the strong acid titration,. This page assumes that you know. Indicators are therefore only useful in the titration of acids. Titration Of Indicators.
From www.slideshare.net
Acid base titration Titration Of Indicators The graph shows the results obtained using two indicators (methyl red and. They are usually weak acids or bases, but their conjugate base or acid. The ph range of phenolphthalein is about 8.3 to 10.0, but the. In the strong acid titration,. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show. Titration Of Indicators.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Titration Of Indicators Indicators are substances whose solutions change color due to changes in ph. In the strong acid titration,. They are usually weak acids or bases, but their conjugate base or acid. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. The graph shows the results. Titration Of Indicators.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Of Indicators In the strong acid titration,. Some plants contain coloring agents that. This page assumes that you know. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and. Titration Of Indicators.
From luz-has-richmond.blogspot.com
How Tknow Which Indicator to Use for Titrations LuzhasRichmond Titration Of Indicators The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. They are usually weak acids or bases, but their conjugate base or acid. Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red and. Some. Titration Of Indicators.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Of Indicators The ph range of phenolphthalein is about 8.3 to 10.0, but the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. In the strong acid titration,. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know. The graph shows the results obtained using two indicators (methyl. Titration Of Indicators.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Of Indicators They are usually weak acids or bases, but their conjugate base or acid. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are therefore only useful in the titration of acids and bases that are sufficiently strong to show a definite break in the titration curve. The graph shows the results obtained using. Titration Of Indicators.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Titration Of Indicators In the strong acid titration,. They are usually weak acids or bases, but their conjugate base or acid. The titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are therefore only useful in the titration of acids. Titration Of Indicators.